- Identification of Tetrachloroethylene Sorption Behaviors in Natural Sorbents Via Sorption Models
Md Abdullah Al Masud·Jiyeon Choi·Won Sik Shin*
School of Architecture, Civil, Environmental and Energy Engineering, Kyungpook National University, Daegu 41566, Republic of Korea
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
A number of different methods have been used for modeling the sorption of volatile organic chlorinated compounds such as tetrachloroethylene/perchloroethylene (PCE). In this study, PCE was adsorbed in several natural sorbents, i.e., Pahokee peat, vermicompost, BionSoil®, and natural soil, in the batch experiments. Several sorption models such as linear, Freundlich, solubility-normalized Freundlich model, and Polanyi-Manes model (PMM) were used to analyze sorption isotherms. The relationship between sorption model parameters, organic carbon content ( foc), and elemental C/N ratio was studied. The organic carbon normalized partition coefficient values (log Koc = 1.50-3.13) in four different sorbents were less than the logarithm of the octanol-water partition coefficient (log Kow = 3.40) of PCE due to high organic carbon contents. The log Koc decreased linearly with log foc and log C/N ratio, but increased linearly with log O/C, log H/C, and log (N+O)/C ratio. Both log KF,oc or log KF,oc decreased linearly with log foc (R2 = 0.88-0.92) and log C/N ratio (R2 = 0.57-0.76), butincreased linearly with log (N+O)/C (R2 = 0.93-0.95). The log qmax,oc decreased linearly as log foc and log C/N increased, whereas it increased with log O/C, log H/C and log (N+O)/C ratios. The log qmax,oc increased linearly with (N+O)/C indicating a strong dependence of qmax,oc on the polarity index. The results showed that PCE sorption behaviors were strongly correlated with the physicochemical properties of soil organic matter (SOM).
Keywords: Elemental ratio, Sorption, Sorbent, Solubility-normalization, Soil organic matter, Tetrachloroethylene
Dense non-aqueous liquids (DNAPLs) are hazardous organic chemicals widely used in industrial processes and include chlorinated solvents, creosotes, coal tar, and poly- chlorinated biphenyls, which are frequently detected in soil and groundwater (Feng et al., 2023a). Chlorinated solvent such as tetrachloroethylene (PCE) has been widely used as an industrial solvent for degreasing metal parts, paint stripping, and plastics production, dry clean fabrics and manufacture other chemicals resulting in significant conta- mination of groundwater and soil by chlorinated organic compounds (COCs) (Yin et al., 2022). PCE is a toxic chemical due to its cytotoxicity, carcinogenicity, and per- sistence in the hyporheic zone and groundwater that may pose serious human health and environmental concerns (Liu et al., 2022). It has been included to the US Environmental Protection Agency's (USEPA) Priority Pollutant List as one of 14 volatile organic compounds (Sekar et al., 2022). As a result, researchers are very interested in developing treat- ment technologies for PCE in the hyporheic zone and groundwater. In the field of environmental technology, hyporheic zones and PCE-contaminated groundwater have also consistently attracted attention.
Chlorinated organic pollutants in aquatic systems have been treated by physical, biochemical, and chemical pro- cesses. Although technologies based on membranes (Rani and Karthikeyan, 2021), extraction (Akinpelu et al., 2019), ozonation (Li et al., 2021), biodegradation (Feng et al., 2023b) and advanced oxidation processes (AOPs) (Al-Masud et al., 2022; Masud et al., 2022) are highly efficient, the operational costs are high. To remove organic pollutants from aquatic environment, sorption is one of the main physical processes (Brusseau et al., 2012; Choi and Shin, 2020). However, the types of adsorbents are crucial for the effectiveness of adsorption toward particular pollutants (Kim et al., 2005; Xiao and Huang, 2011). Under anaerobic conditions, reductive dichlorination is carried out biologi- cally by dehalococcoides and chemically by Fe0 (Devore et al., 2022; Dutta et al., 2022).
Sorption is the most important processes controlling interaction between hydrophobic organic contaminants and soils/sediments (Prajapati et al., 2022). Numerous studies have shown that the fate and transport of hydrophobic organic compounds (HOCs) in soil is highly dependent on sorption/desorption characteristics (Christensen et al., 2022). A few studies have reported on the application of solubility-normalized model to explain nonlinear sorption of HOCs in soils and sediments (Allen-King et al., 2002; Carmo et al., 2000; Kleineidam et al., 2002; Masud and Shin, 2022; Qi et al., 2004; Ran et al., 2004). The purpose of this study is to investigate the sorption and desorption behaviors of PCE in several natural sorbents. An accurate estimation of sorption may have wide implications on the remediation of HOCs in natural environments. In this paper, sorption/desorption characteristics of PEC in natural sorbents with wide range of organic carbon content.
This study examines the details of the sorption of PCE on four different sorbents, i.e., Pahokee peat, vermicompost, BionSoil®, and natural soil through batch experiments. The sorption behaviors of PCE in natural sorbents were analyzed using several models such as linear, Freundlich, solubility-normalized Freundlich and Polanyi-Manes (PM) models. The objectives of this study were to compare the sorption of different sorbents and compare different models for sorption with sorbents. The relationship between sorption model parameters, organic carbon content ( foc), and elemental C/N ratio was studied.
2.1. Chemicals
Radiolabeled [1,2-14C] PCE (ChemSyn Laboratories, 2.2 mCi/mmol, >99.9%) were obtained from ARC chemicals (Saint Louis, MO, USA) and used as radiotracers (Kan et al., 1998, 1997). The [14C] compounds were further diluted with unlabeled tetrachloroethylene (PCE, >99.9%, HPLC grade, Sigma-Aldrich, Germany) stock solutions (1,000 mg L-1 in methanol, HPLC grade, Sigma-Aldrich, Germany) to yield desired concentrations. Sodium azide (NaN3, 99%, Daejung Chemical Co., Siheung, Korea) was added to the solution as a bacterial inhibitor. PCE solution was prepared before each sorption experiment using the 14C stock solution. The physicochemical properties of PCE are sum- marized in Table 1. Electrolyte solution used in experiments was prepared in distilled and deionized water (DDI, MilliporeSigma™ Synergy™, Thermo Fisher Scientific, Waltham, MA, USA) containing 1.0 mM of CaCl2·2H2O (99%, Daejung Chemical Co., Siheung, Korea), 0.5 mM of MgCl2, (98%, Duksan, Ansan, Korea) and 1 mM Na2B4O7· 10H2O (98%, Duksan Chemical Co., Ansan, Korea).
2.2. Sorbents
Four different carbon-based sorbents such as Pahokee peat (International Humic Substances Society, USA), BionSoil® (Bion Environmental Technologies, Inc., USA), vermicom- post (Eco Biotech, Korea), and a natural soil (collected from a local horizon near Daegu, Korea) were chosen for to investigate the sorption behavior of PCE. The sorbent characteristics analyzed by Huffmann Laboratories; Inc. (Golden, CO, USA) was listed in Table 2. The organic carbon content ( foc) was in the order of Pahokee peat (44.6 wt%) > vermicompost (13.7 wt%) > BionSoil® (11.12 wt%) > natural soil (2.25 wt%). The sorbent samples were air-dried, sieved through a U.S. Sieve Series No. 20 sieve and kept in gas-tight amber bottle until use.
2.3. Experimental procedures
Sorption experiments were conducted using 40-mL amber vials (Fisher Co.) with open-top polypropylene screw thread caps with Teflon-faced silicon septa (Kimble Chase, USA). Different weights (g) of sorbents are used in this study because sorbents have widely different sorption capacities. Predetermined amounts of sorbents such as Pahokee peat (0.02 g), vermicompost (0.03 g), BionSoil® (0.03 g) and natural soil (0.1 g), were transferred to the vial before the addition of PCE solution. To obtain sorption isotherms, eight different initial concentrations (from 10 mg L-1 to 100 mg L-1) of PCE solution were used. The vials containing the sorbent was filled with the spiking solution minimizing the heads- pace. Control vials without sorbents were also prepared in duplicate. The mixture was horizontally mixed at 150 rpm on an orbital shaker at room temperature. After 2 days, the soil was separated from the solution by centrifugation at 2,500 rpm for 20 minutes. 1.0 mL of the supernatant was mixed with 10 mL of scintillation cocktail (Ultima Gold, Sigma) and the radioactivity was analyzed by liquid sci- ntillation counter (LSC, EG&G Wallac Co., 1220 Quantulus). Approximately 95% of the supernatant was gently removed using a pipette and spiked with solute-free electrolyte solution for sorption. The actual amount of supernatant removed and added was determined gravimetrically. After 2 days, the sorbent/water mixture was centrifuged at 2,500 rpm for 20 minutes and the radioactivity of the supernatant was analyzed. The sorbed PCE concentrations were calculated by assuming all concentration changes in solution phase result from sorption onto the solid phase. All experiments were run in duplicate.
2.4. Sorption models
2.4.1. Linear Model
Linear sorption model was used to analyze sorption and desorption isotherms:

where q (mg/kg) is total concentration of sorbed compound in the soil phase, Kp (L/kg) is linear partition coefficient, C (mg/L) is chemical concentration in solution phase, Koc (= Kp /foc, L/kg OC) is organic carbon normalized partition coefficient, and foc is organic carbon content, respectively.
To evaluate the relative sorption affinity of PCE in the sorbents, sorption affinity ratio (SAR) is defined as:
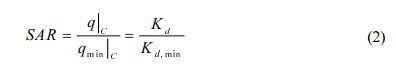
where q|C and qmin|C are the solid phase concentration of a sorbent and that of a sorbent with minimum sorption affinity (natural soil) at aqueous phase concentration C, respectively. The Kd and Kd, min are the distribution coefficient of each sorbent, respectively.
2.4.2. Freundlich Model
Freundlich model was also used to fit the sorption and desorption data:
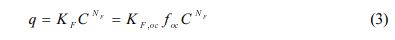
where KF [(mg/kg)/(mg/L)N] and NF (dimensionless) are Freundlich sorption coefficient and the Freundlich exponent and KF, oc [(mg/kg OC)/(mg/L)N] is organic carbon normalized Freundlich sorption coefficient, respectively.
2.4.3. Solubility-normalized Freundlich model
In this study, a solubility-normalized Freundlich model was used to fit the single-solute sorption data (Allen-King et al., 2002; Carmo et al., 2000; Kile et al., 1999):
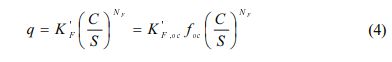
where S (mg/L) is the solute’s aqueous solubility, K'F (mg/kg) is the unit-normalized Freundlich sorption coefficient and K'F (mg/kg OC) is organic carbon normalized form of K'F. The use of C/S yields that K'F values are inde- pendent of the values of NF. In Eq. (3), K'F has the same units as q and its magnitude is equal to the value of q at C/S = 1. Thus K'F represents the mass of HOC sorbed per unit mass of sorbent when the C approaches saturation (C → S), regardless of the units of C. This approach implicates that the unit-normalized Freundlich sorption coefficient (K'F) has both physical significance and meaningful units in addition to providing a flexible choice of units for C.
2.4.4. Polanyi-Manes model (PMM)
Polanyi-Manes (PDM) model was also used to fit sorption data (Allen-King et al., 2002; Carmo et al., 2000; Chiou and Kile, 1998; Kile et al., 1999):

where qmax is the maximum sorption capacity (mg/kg), a and β are fitting parameters, Vm is the molar volume of the solute (L/mol) and εsw is the solute sorption potential (= RT ln(S/C)). To decrease the number of parameters to be esti- mated, b was fixed to be 2.0 (Kleineidam et al., 2002):
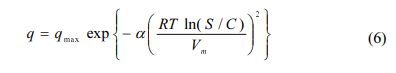
The sorption model parameters were determined by using a commercial software package, Table Curve 2D® (Version 5.1, SPSS, Inc.).
|
Table 1 Physicochemical properties of PCE |

Data obtained from SRC PhysProp database (http://www.syrres.com). |
|
Table 2 Summary of soil characteristics analyzed by Huffman Laboratories, Inc. (Golden, CO, USA). Data indicates percentage per g of dry soil |
3.1. Sorption isotherm
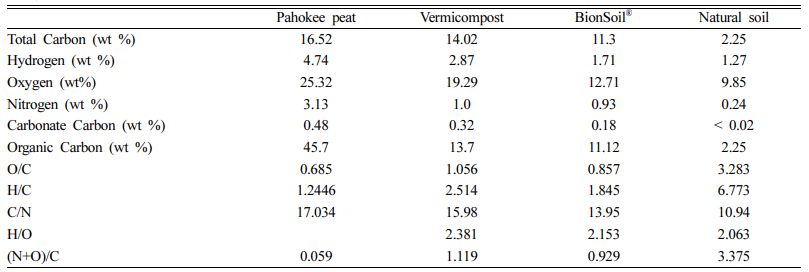
The relationship between elemental ratios of natural sorbents: H/C, (N+O)/C and C/N versus O/C was shown in Fig. 1. The H/C, (N+O)/C ratios are linearly increasing with O/C ratio, whereas linearly decreasing with C/N ratio.
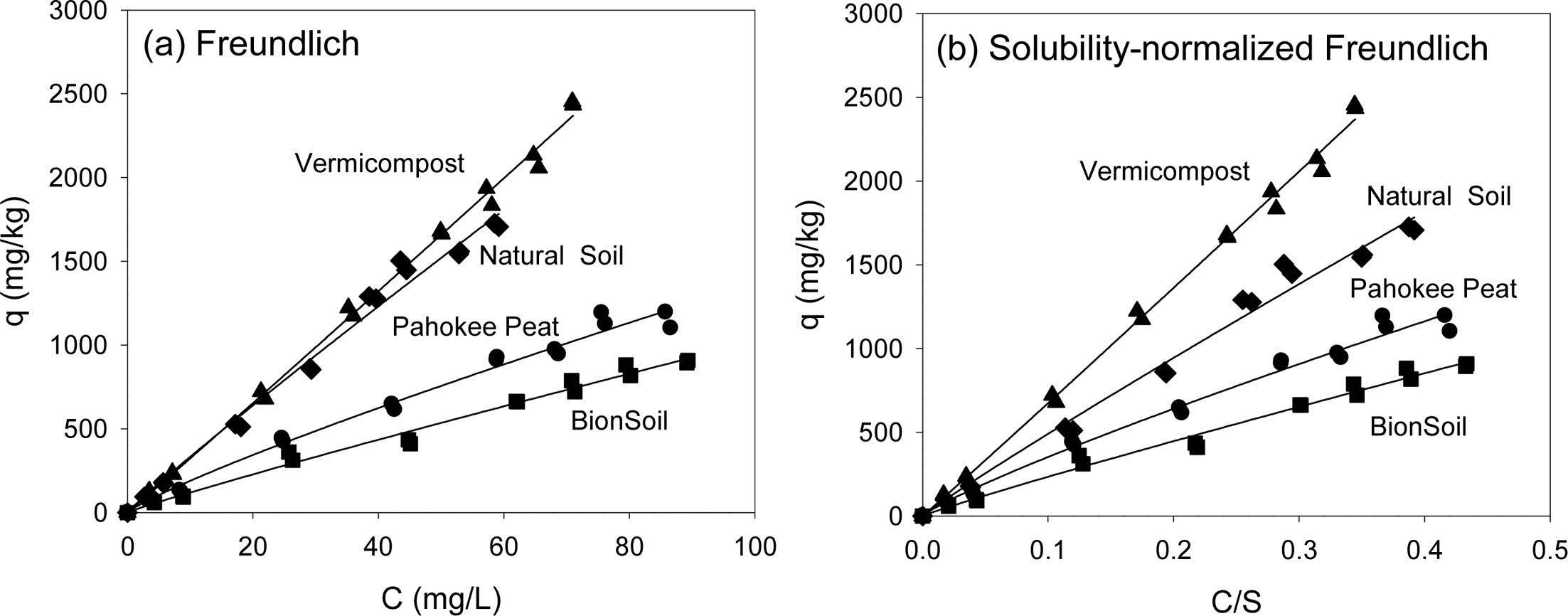
The sorption isotherm of PCE on natural sorbents was presented in Fig. 2. The linear, Freundlich, and solubility-normalized Freundlich models were fitted well to the sorption data (0.947 < R2 < 0.996). As listed in Table 3, the log Koc value was in the order of natural soil > vermi- compost > Pahokee peat > BionSoil® for sorption of PCE. The log Koc values (1.50-3.13) in four different sorbents were less than log Kow (= 3.40) of PCE. The order of increase in log Koc value was not correlated with the log-transformed organic carbon content (log foc), atomic ratios (log O/C, log C/N log H/C, and log (N+O)/C). The difference in log Koc value of the soils is presumably due to diagenetically altered soil properties; diagenetically ‘older’ organic carbon appears to cause stronger bonding in the natural soil (Cornelissen et al., 2005, 2004).
As listed in Table 3, the SAR was in the order of natural soil = vermicompost > Pahokee peat > BionSoil®. This suggests that diagenetically ‘older’ natural soil has higher SAR than the others.
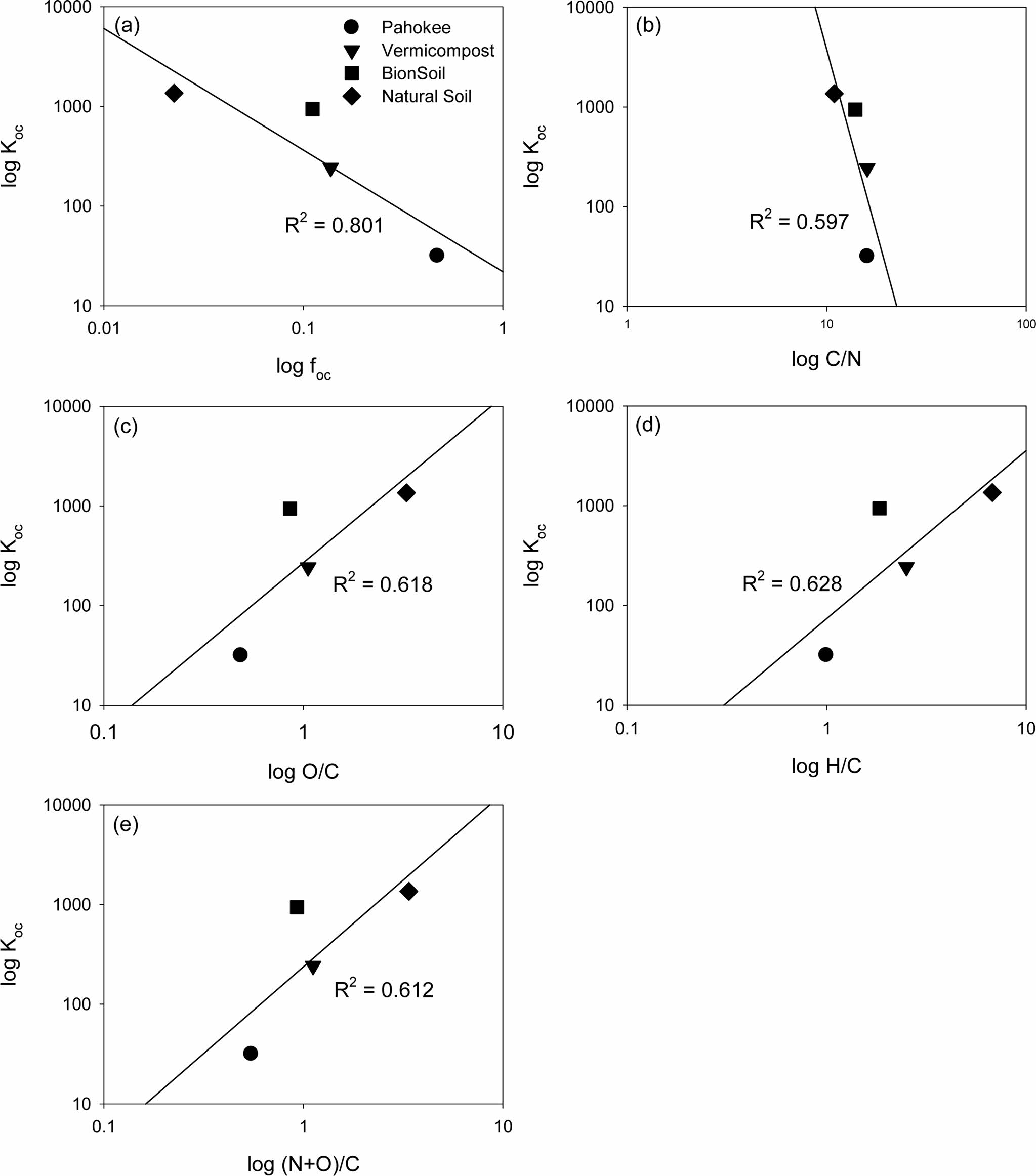
The relationships between the log Koc vs. log foc and log-transformed atomic ratios (log C/N, log O/C, log H/C and log (N+O/C)) were shown in Fig. 3. The log Koc decreased linearly with log foc and log C/N ratio. In contrast, the log Koc increased linearly with log O/C, log H/C and also log (N + O)/C ratio (Fig. 3).
3.2. Freundlich and Solubility-normalized Freundlich model
The Freundlich model parameters are listed in Table 4. As indicated by the R2 values (0.950–0.996), the Freundlich model fitted well to the sorption data. The Freundlich sorption constant, KF, indicating the sorption capacity of the sorbent was in the order of natural soil > vermicompost > Pahokee peat > BionSoil® for sorption. The organic carbon-normalized Freundlich coefficient (KF,oc) was also in the order of natural soil > vermicompost > Pahokee peat > BionSoil®.
As indicated by the NFvalues (0.93–1.02) of the Freun- dlich model, sorption of PCE were nearly linear, except Pahokee peat (0.86). The Freundlich exponent, NF,is a measure of the deviation from linearity of the sorption. If a value for NF is equal to unity, the sorption is linear (Choi and Shin, 2020; Shin and Song, 2005). If NF value is above 1, this implies that sorption process is chemical, but if NF value is below 1, sorption is favorable, a physical process (Masud et al., 2023). The NF values at equilibrium represent favo- rable sorption, and therefore this would seem to suggest that physical, which is referred the sorption bond becomes weak and conducted with van der Waals forces (Özcan et al., 2006). As listed in Table 4, PCE sorption was favorable in Pahokee peat, BionSoil®, and natural soil (i.e., NF < 1), except vermicompost that showed NF value slightly above 1.
As listed in Table 5, the solubility-normalized Freundlich coefficient (K'F) was in the order of vermicompost > natural soil > Pahokee peat > BionSoil®. The advantage of the solubility-normalized Freundlich model is that the K'F value represents the mass of HOC sorbed per unit mass of sorbent when the C concentration approaches saturation (C → S), regardless of the units of C. The effect of sorbate charac- teristics on sorption is clearly evident at low equilibrium concentrations. To examine this, the q value for an arbitrary value of C = 20 mg/L (q|C=20) was calculated by using Eq. (4) and the unit-normalized Freundlich sorption coefficients (K'F and K'F,oc) listed in Table 5. The calculated q|C=20 values for PCE were in the order of vermicompost > natural soil > Pahokee peat > BionSoil®. When organic carbon normalized, the K'F,oc was in the order of natural soil >> vermicompost > BionSoil® > Pahokee peat. This explains that geologically weathered soil (i.e., natural soil) has higher K'F,oc value and thus higher qe (mg/kg OC) value.
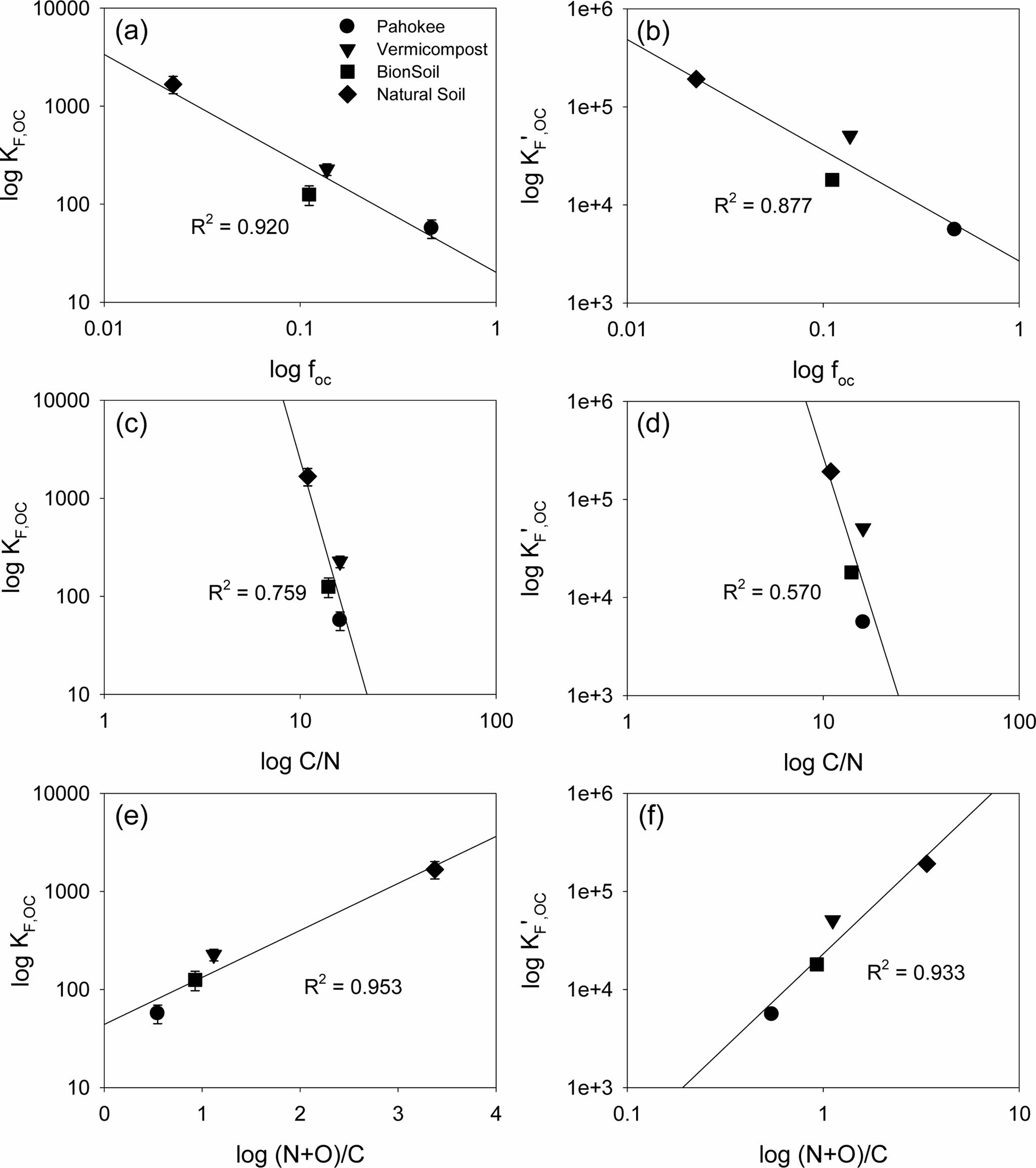
The relationships between the log KF,oc or log K'F,oc vs. log foc and log-transformed atomic ratios (log C/N and log (N+O/C)) were shown in Fig. 4. Both log KF,oc or log K'F,oc decreased linearly with log foc (R2 = 0.88-0.92) and log C/N ratio (R2 = 0.57-0.76), but increased linearly with log (N + O)/C (R2 = 0.93-0.95).
3.3. Polanyi-Dubinin-Manes (PDM) model
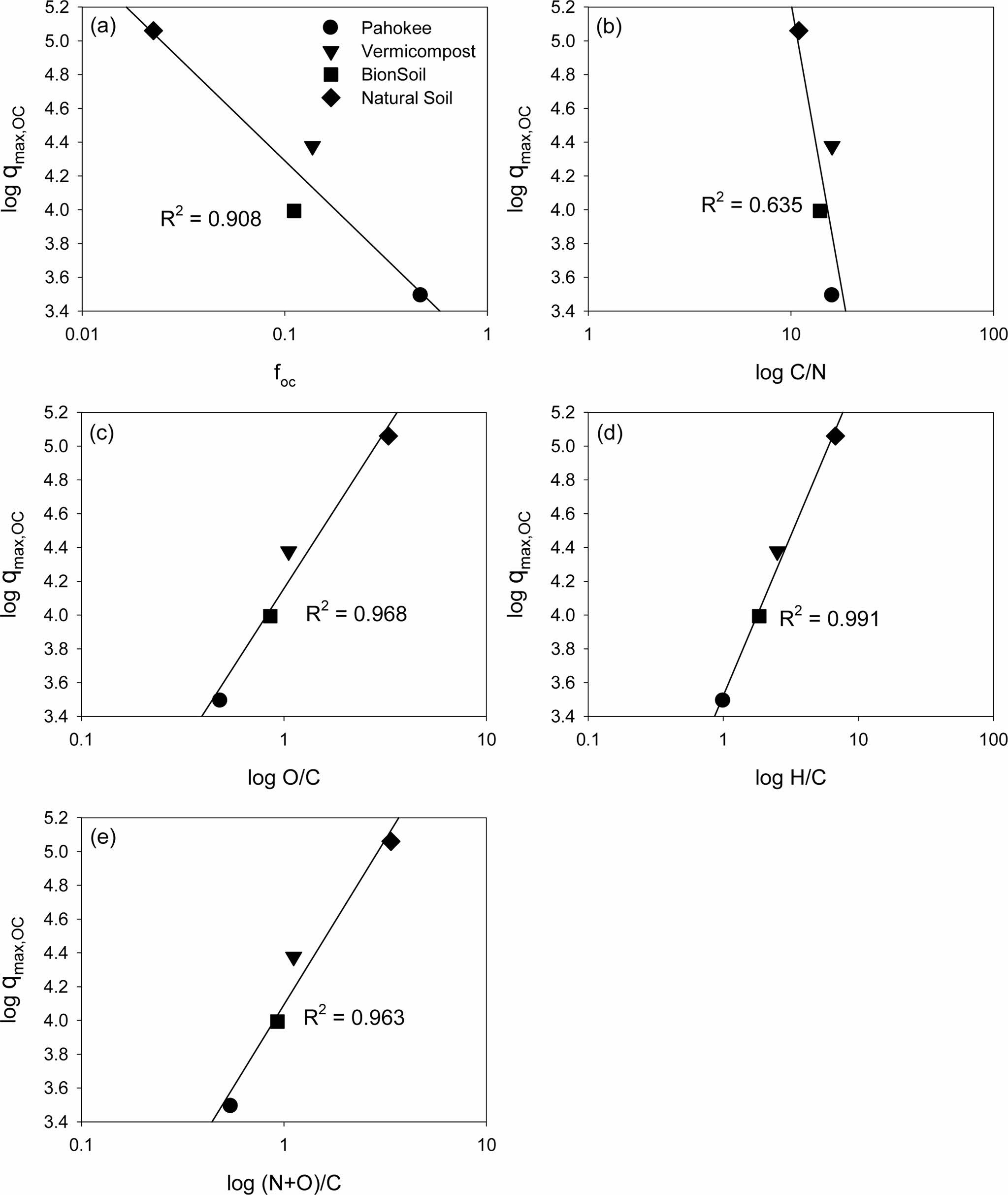
The results of PDM model analysis are listed in Table 6. The maximum sorption capacity (qmax) was in the order of vermicompost > natural soil > Pahokee peat > BionSoil®. The organic carbon-normalized maximum sorption capacity (qmax,oc) was in the order of natural soil > vermicompost > Pahokee peat > BionSoil®. In addition, qmax,oc rather than qmax was able to explain higher maximum sorption capacity in geologically ‘older’ soil. The relationships between log qmax,oc and log foc and log-transformed elemental ratios (log C/N, log O/C, log H/C and log (N+O)/C) were shown in Fig. 5. The log qmax,oc decreased linearly as log foc and log C/N increased, whereas increased with log O/C, log H/C and log (N+O)/C ratios. The log qmax,oc increased linearly with (N+O)/C indicating that a strong dependence of qmax,oc on the polarity index.
3.4. Solubility normalization
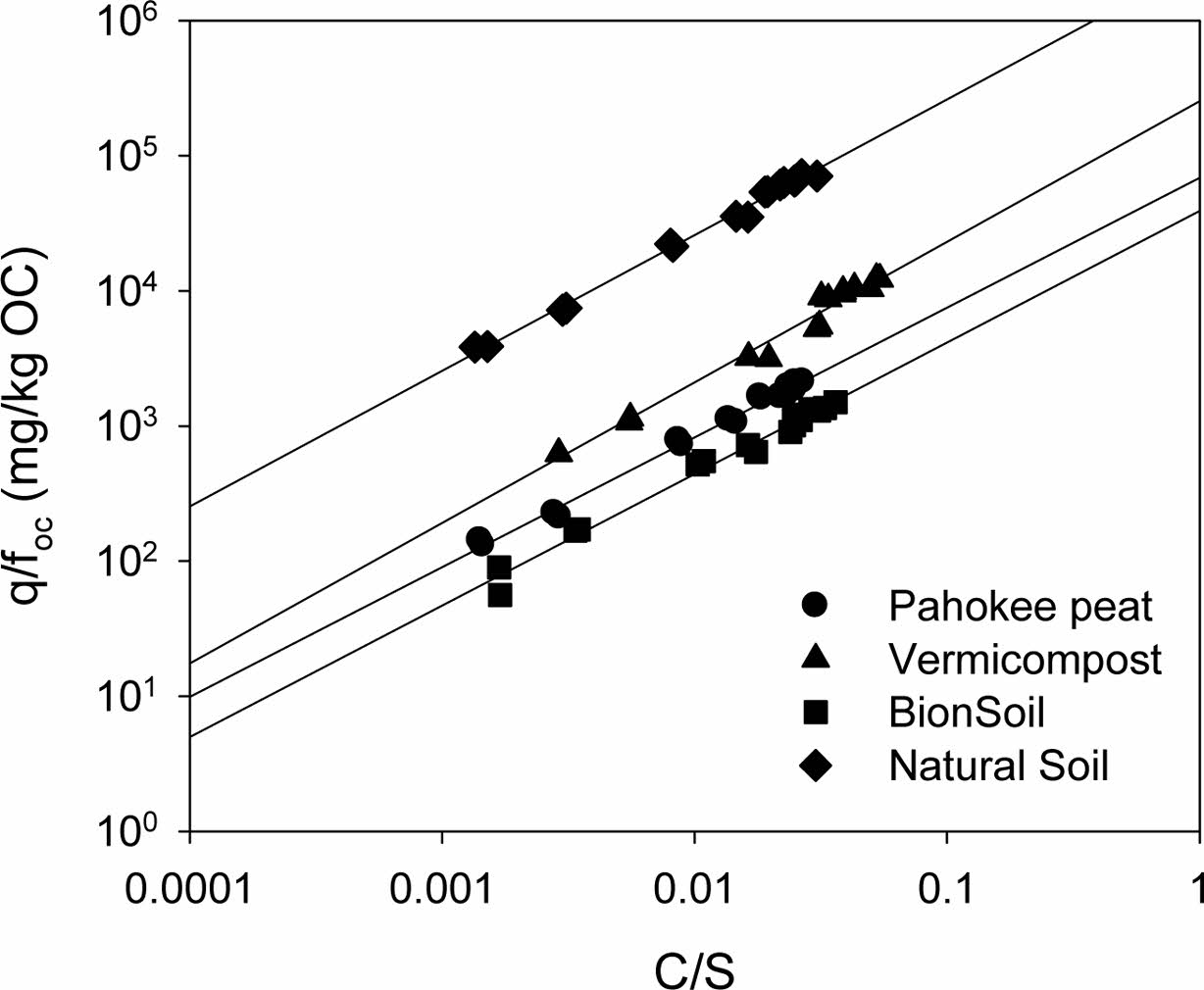
As shown in Fig. 6, provides the relationship between q/foc versus C/S for PCE sorption onto the sorbents. If Eq. (3) and Eq. (4) are normalized by organic carbon content (Allen-King et al., 2002; Shin and Song, 2005):
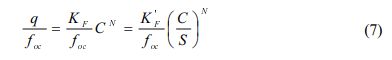
As concentration approaches solubility (C/S → 1), q/foc is equal to q/foc at C/S = 1 satisfying Raoult’s law. At C = S, K'F/foc provides a measure of q at aqueous solubility (the highest possible aqueous concentration). As depicted in Fig. 6 and summarized in Table 7, K'F/foc (= q/foc) at C → S was in the order of natural soil > vermicompost > Pahokee peat > BionSoil®.
When C is very small (i.e., C → 1 mg/L), Eq. (7) becomes:
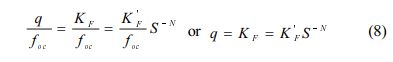
The values of KF (=K'F S-N) is an estimate of q at low aqueous concentration satisfying Henry’s law. Then the solubility-normalized Freundlich isotherm elucidates that the relationship between KF (at C = 1 mg/L) and SN. As shown in Fig. 6 and summarized in Table 7, K'F/foc (= q/foc) at C → 1 mg/Lwas also in the order of natural soil > vermicompost > Pahokee peat > BionSoil®, consistent with the order at C → S. In addition, the order of increases in K'F/foc values at both C → S and C1 mg/Lwere consistent with K'F,oc and qmax,oc. Therefore, it is concluded that solubility-normali- zation is useful in elucidating sorption capacities in soils with widely different characteristics (i.e., foc and elemental ratios).
|
Fig. 1 The relationship between elemental ratios of several sorbents: H/C, (N+O)/C and C/N versus O/C. |
|
Fig. 2 Sorption isotherms of PCE in several sorbents. (a) Freundlich and (b) solubility-normalized Freundlich isotherm |
|
Fig. 3 Relationship between linear sorption model parameter (Koc) and organic carbon content ( foc) or elemental ratios (C/N, O/C, H/C, and (N+O)/C) |
|
Fig. 4 Relationship between organic carbon-normalized Freundlich (KF,oc) and solubility-normalized Freundlich ( K'F,oc) sorption coefficients and organic carbon content ( foc) or elemental ratios (C/N and (N+O)/C). |
|
Fig. 5 Relationship between PDM parameter (qmax,oc) and organic carbon content ( foc) or elemental ratios (C/N, O/C, H/C, and (N+O)/C). |
|
Fig. 6 Relationship between q/foc (= /foc) and C/S for sorption of PCE in natural sorbents. Note that qoc/foc= /foc as concentration approaches solubility (C/S → 1). |
|
Table 3 Linear model parameters for PCE sorption in several sorbents. (Koc = organic carbon normalized partition coefficient) |
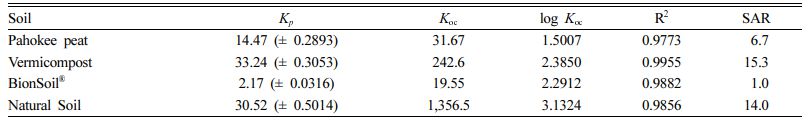
Units: Kp = (mg/kg)/(mg/L) and Koc = (mg/kg OC)/(mg/L). Number in parenthesis indicates standard deviation. |
|
Table 4 Freundlich model parameters for PCE sorption in several sorbents |
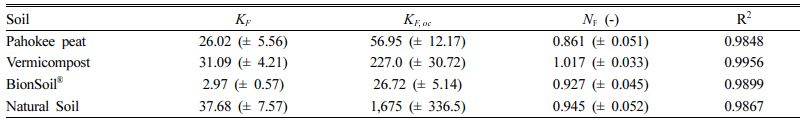
Units: KF = [(mg/kg)/(mg/L)N], KF,oc = [(mg/kg OC)/(mg/L)N], NF = dimensionless. Number in parenthesis indicates standard deviation. |
|
Table 5 Solubility-normalized Freundlich model parameters for PCE sorption in several sorbent |
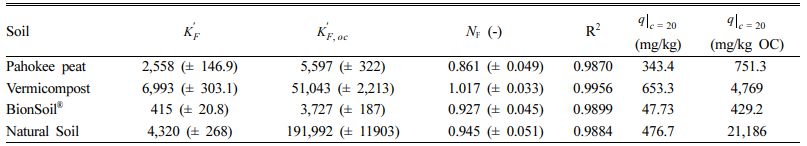
Units: K'F = (mg/kg), K'F,oc = (mg/kg OC), N = dimensionless. Number in parenthesis indicates standard deviation |
|
Table 6 PDM model parameters for PCE sorption in several sorbents |

Units: qmax = (mg/kg)/(mg/L), qmax, oc = (mg/kg OC)/(mg/L) and a = constant. Number in parenthesis indicates standard deviation. |
|
Table 7 The q/foc values for Henry’s law (at C = 1 mg/L) and Raouls’t law(C→ S) regions |
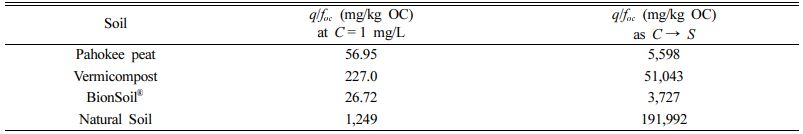
Note that q/foc = K'F/foc (mg/g OC) at high concentration (as C → S) and q/foc = KF/foc (mg/g OC) at low absolute concentration (C = 1 mg/L). |
Sorption characteristics of PCE in several sorbents with widely different organic carbon contents were investigated. To characterize the sorption behaviors of PCE, four different models were used, i.e., linear, Freundlich, solubility-normalized Freundlich, and Polanyi-Manes. The parameters of the sorption model, the organic carbon content (foc), and the elemental C/N ratio were investigated. Due to the high organic carbon contents, the log Koc values in four distinct sorbents were lower than the log Kow of PCE. The log Koc decreased linearly with log foc and log C/N ratio, but increased linearly with log O/C, log H/C and also log (N+O)/C ratio. Both log KF,oc or log K'F,oc decreased linearly with log foc and log C/N ratio, butincreased linearly with log (N+O)/C. The log qmax,oc decreased linearly as log foc and log C/N increased, whereas increased with log O/C, log H/C and log (N+O)/C ratios. The log qmax,oc increased linearly with (N+O)/C that a strong dependence of qmax,oc on the polarity index. For a better mechanistic explanation of the sorption behavior of PCE onto Pahokee peat, vermicompost, BionSoil®, and natural soil sorbents, it is necessary to analyze Brunauer-Emmett-Teller (BET), Fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), and X-ray diffraction (XRD) analysis, as suggested in the further study.
This work was supported by Korea Environmental Industry & Technology Institute (KEITI) through Aquatic Ecosystem Conservation Research Program, funded by Korea Ministry of Environment (MOE) (No. 2021003040004).
- 1. Akinpelu, A.A., Ali, M.E., Johan, M.R., Saidur, R., Qurban, M.A., and Saleh, T.A., 2019, Polycyclic aromatic hydrocarbons extraction and removal from wastewater by carbon nanotubes: A review of the current technologies, challenges and prospects, Process Saf. Environ. Prot. 122, 68-82. https://doi.org/10.1016/j.psep.2018.11.006
-

- 2. Al-Masud, M.A., Kim, D.G., and Shin, W.S., 2022, Highly efficient degradation of phenolic compounds by Fe(II)-activated dual oxidant (persulfate/calcium peroxide) system, Chemosphere, 299, 134392. https://doi.org/10.1016/j.chemosphere.2022. 134392
-

- 3. Allen-King, R.M., Grathwohl, P., and Ball, W.P., 2002, New modeling paradigms for the sorption of hydrophobic organic chemicals to heterogeneous carbonaceous matter in soils, sediments, and rocks, Adv. Water Resour., 25(8-12), 985-1016. https://doi.org/10.1016/S0309-1708(02)00045-3
-

- 4. Brusseau, M.L., Schnaar, G., Johnson, G.R., and Russo, A.E., 2012, Nonideal transport of contaminants in heterogeneous porous media: 10. Impact of co-solutes on sorption by porous media with low organic-carbon contents, Chemosphere, 89, 1302-1306. https://doi.org/10.1016/j.chemosphere.2012.05.027
-

- 5. Carmo, A.M., Hundal, L.S., and Thompson, M.L., 2000, Sorption of hydrophobic organic compounds by soil materials: Application of unit equivalent Freundlich coefficients, Environ. Sci. Technol., 34(20), 4363-4369. https://doi.org/10.1021/es000 968v
-

- 6. Chiou, C.T. and Kile, D.E., 1998, Deviations from sorption linearity on soils of polar and nonpolar organic compounds at low relative concentrations, Environ. Sci. Technol. 32(3), 338-343. https://doi.org/10.1021/es970608g
-

- 7. Choi, J. and Shin, W.S., 2020, Removal of salicylic and ibuprofen by hexadecyltrimethylammonium-modified montmorillonite and zeolite, Minerals, 10(10), 898, 1-15. https://doi.org/10. 3390/min10100898
-

- 8. Christensen, E.R., Wang, Y., Huo, J., and Li, A., 2022, Properties and fate and transport of persistent and mobile polar organic water pollutants: A review, J. Environ. Chem. Eng., 10(2), 107201. https://doi.org/10.1016/j.jece.2022.107201
-

- 9. Cornelissen, G., Gustafsson, Ö., Bucheli, T.D., Jonker, M.T.O., Koelmans, A.A., and Van Noort, P.C.M., 2005, Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: Mechanisms and consequences for distribution, bioaccumulation, and biodegradation, Environ. Sci. Technol., 39(18), 6881-6895. https://doi.org/10.1021/es050191b
-

- 10. Cornelissen, G., Kukulska, Z., Kalaitzidis, S., Christanis, K., and Gustafsson, Ö., 2004, Relations between environmental black carbon sorption and geochemical sorbent characteristics, Environ. Sci. Technol., 38(13), 3632-3640. https://doi.org/10.1021/es0498742
-

- 11. Devore, C.L., Rodriguez-Freire, L., Villa, N., Soleimanifar, M., Gonzalez-Estrella, J., Ali, A.M.S., Lezama-Pacheco, J., Ducheneaux, C., and Cerrato, J.M., 2022, Mobilization of As, Fe, and Mn from contaminated sediment in aerobic and anaerobic conditions: Chemical or microbiological triggers?, ACS Earth Space Chem., 6(7), 1644-1654. https://doi.org/10.1021/acsearthspacechem. 1c00370
-

- 12. Dutta, N., Usman, M., Ashraf, M.A., Luo, G., and Zhang, S., 2022, A critical review of recent advances in the bio-remediation of chlorinated substances by microbial dechlorinators, Chem. Eng. J. Adv., 12, 100359. https://doi.org/10.1016/j.ceja. 2022.100359
-

- 13. Feng, C., Liu, F., Huang, F., Chen, L., and Bi, E., 2023a, Dense nonaqueous phase liquids back diffusion controlled by biodegradation and heterogeneous sorption-desorption, J. Clean. Prod., 382, 135370. https://doi.org/10.1016/j.jclepro.2022.135370
-

- 14. Feng, C., Liu, F., Huang, F., Chen, L., and Bi, E., 2023b, Dense nonaqueous phase liquids back diffusion controlled by biodegradation and heterogeneous sorption-desorption, J. Clean. Prod., 382, 135370. https://doi.org/10.1016/j.jclepro.2022.135370
-

- 15. Kan, A.T., Fu, G., Hunter, M., Chen, W., Ward, C.H., and Tomson, M.B., 1998. Irreversible sorption of neutral hydrocarbons to sediments: Experimental observations and model predictions, Environ. Sci. Technol., 32(7), 892-902. https://doi.org/10.1021/es9705809
-

- 16. Kan, A.T., Fu, G., Hunter, M.A., and Tomson, M.B., 1997, Irreversible adsorption of naphthalene and tetrachiorobiphenyl to Lula and surrogate sediments, Environ. Sci. Technol., 31(8), 2176-2185. https://doi.org/10.1021/es9601954
-

- 17. Kile, D.E., Wershaw, R.L., and Chiou, C.T., 1999, Correlation of soil and sediment organic matter polarity to aqueous sorption of nonionic compounds, Environ. Sci. Technol., 33(12), 2053-2056. https://doi.org/10.1021/es980816o
-

- 18. Kim, J.-H., Shin, W.S., Song, D.-I., and Choi, S.J., 2005, Multi-step competitive sorption and desorption of chlorophenols in surfactant modified montmorillonite, Water. Air. Soil Pollut., 166, 367-380. https://doi.org/10.1007/s11270-005-6329-5
-

- 19. Kleineidam, S., Schüth, C., and Grathwohl, P., 2002, Solubility-normalized combined adsorption-partitioning sorption isotherms for organic pollutants, Environ. Sci. Technol., 36(21), 4689-4697. https://doi.org/10.1021/es010293b
-

- 20. Li, W., Zhu, N., Yuan, H., and Shen, Y., 2021, Influence of sludge organic matter on elimination of polycyclic aromatic hydrocarbons (PAHs) from waste activated sludge by ozonation: Controversy over aromatic compounds, Sci. Total Environ., 797, 149232. https://doi.org/10.1016/j.scitotenv.2021.149232
-

- 21. Liu, S., Yan, E.Z., Turyk, M.E., Katta, S.S., Rasti, A.F., Lee, J.H., Alajlouni, M., Wallace, T.E., Catt, W., and Aikins, E.A., 2022, A pilot study characterizing tetrachloroethylene exposure with exhaled breath in an impacted community, Environ. Pollut., 297, 118756. https://doi.org/10.1016/j.envpol.2021.118756
-

- 22. Masud, M.A. Al, Kim, D.G., and Shin, W.S., 2022, Degradation of phenol using Fe(II)-activated CaO2: effect of ball-milled activated carbon (ACBM) addition, Environ. Res., 214, 113882. https://doi.org/10.1016/j.envres.2022.113882
-

- 23. Masud M. A. A., and Shin W. S. 2022, Single and binary competitive sorption of phenanthrene and pyrene in natural and syntheic sorbents, J. Soil Groundwater Environ., 27(6), 11-21. https://doi.org/10.7857/JSGE.2022.27.6.011
-

- 24. Masud, M.A. Al, Shin, W.S., and Kim, D.G., 2023, Degradation of phenol by ball-milled activated carbon (ACBM) activated dual oxidant (persulfate/calcium peroxide) system: Effect of preadsorption and sequential injection, Chemosphere, 312, 137120. https://doi.org/10.1016/j.chemosphere.2022.137120
-

- 25. Özcan, A., Öncü, E.M., and Özcan, A.S., 2006, Kinetics, isotherm and thermodynamic studies of adsorption of Acid Blue 193 from aqueous solutions onto natural sepiolite, Colloids Surfaces A Physicochem. Eng. Asp., 277, 90-97. https://doi.org/10. 1016/j.colsurfa.2005.11.017
-

- 26. Prajapati, A., Narayan Vaidya, A., and Kumar, A.R., 2022, Microplastic properties and their interaction with hydrophobic organic contaminants: a review, Environ. Sci. Pollut. Res., 29, 49490-49512. https://doi.org/10.1007/s11356-022-20723-y
-

- 27. Qi, S., 2004, Comment on ¡°Sorption nonlinearity for organic contaminants with diesel soot: Method development and isotherm interpretation¡±, Environ. Sci. Technol., 38(20), 5485. https://doi.org/10. 1021/es0404771
-

- 28. Ran, Y., Xing, B., Rao, P.S.C., and Fu, J., 2004, Importance of adsorption (hole-filling) mechanism for hydrophobic organic contaminants on an aquifer kerogen isolate, Environ. Sci. Technol., 38(16), 4340-4348. https://doi.org/10.1021/es035168+
-

- 29. Rani, C.N. and Karthikeyan, S., 2021, Synergic effects on degradation of a mixture of polycyclic aromatic hydrocarbons in a UV slurry photocatalytic membrane reactor and its cost estimation, Chem. Eng. Process. - Process Intensif., 159, 108179. https://doi.org/10.1016/j.cep.2020.108179
-

- 30. Sekar, A., Varghese, G.K., and Varma, R., 2022, Exposure to volatile organic compounds and associated health risk among workers in lignite mines, Int. J. Environ. Sci. Technol., https://doi.org/10.1007/s13762-022-04056-4
-

- 31. Shin, W.S. and Song, D.I., 2005, Solubility-normalized Freundlich isotherm for the prediction of sorption of phenols in HDTMA modified montmorillonite, Geosci. J., 9, 249-259. https://doi.org/10.1007/BF02910585
-

- 32. Xiao, B. and Huang, W., 2011, The equilibria of bisolute sorption on soil, Chemosphere, 83(7), 1005-1013. https://doi.org/10. 1016/j.chemosphere.2011.02.009
-

- 33. Yin, X., Hua, H., Dyer, J., Landis, R., Fennell, D., and Axe, L., 2023, Degradation of chlorinated solvents with reactive iron minerals in subsurface sediments from redox transition zones, J. Hazard. Mater., 445, 130470. https://doi.org/10.1016/j.jhazmat. 2022. 130470
-

 This Article
This Article
-
2022; 27(6): 47-57
Published on Dec 31, 2022
- 10.7857/JSGE.2022.27.6.047
- Received on Dec 6, 2022
- Revised on Dec 16, 2022
- Accepted on Dec 21, 2022
 Services
Services
- Abstract
1. introduction
2. materials and methods
3. results and discussion
4. conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Won Sik Shin
-
School of Architecture, Civil, Environmental and Energy Engineering, Kyungpook National University, Daegu 41566, Republic of Korea
- E-mail: wshin@knu.ac.kr





