- Effects of NaOH Treatment on the Adsorption Ability of Surface Oxidized Activated Carbon for Heavy Metals
Min-Ho Park·So-Jeong Kim·Jung Hwan Kim·Jae-Woo Park*
Department of Civil and Environmental Engineering, Hanyang University, Seoul 04763, Korea
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Heavy metal (Zinc, Cadmium, Lead) adsorption onto surface modified activated carbon was performed in order to better understand the effect of sodium ion addition to activated carbon. Surface modification methods in this research included water washing, nitric acid washing, and sodium addition after nitric acid washing. These surface modifications generated oxygen functional groups with sodium ions on the surface of the activated carbon.. This caused the change of the specific surface area as well as in the ratio of the carboxyl groups. Heavy metal adsorption onto sodium-containing activated carbon was the most among the three modifications. After the adsorption of heavy metals, the carboxyl group ratio decreased and sodium ions on the surface of the activated carbon were almost non-existent after the adsorption of heavy metals onto sodium-containing activated carbon. The results from this research indicated that ion exchange with sodium ions in carboxyl groups effectively improved heavy metal adsorption rather than electrostatic adsorption and hydrogen ion exchange.
Keywords: Activated carbon, Heavy metal, Functional group, Ion exchange, Electrostatic interaction, Adsorption
Carbon materials, such as activated carbon, biochar, graphite oxide, and so on, have been applied toward the adsorption of various environmental contaminants (Babel and Kurniawan, 2003; Ismadji et al., 2005). Activated carbon is widely used as a gas or liquid adsorbent, catalyst support, and catalyst due to its low price, excellent adsorp- tion, and low regeneration costs compared to the other porous materials, such as zeolite and silica (Kim et al., 2011; Wang et al., 2022). Activated carbon can adsorb organic compounds, but heavy metals are not adsorbed as strongly as organic contaminants (Babel and Kurniawan, 2003). It is possible to increase the adsorption of specific chemical substances by modifying the activated carbon surface. Surface-modified activated carbon can adsorb heavy metals via electric double layers (EDL), ion exchange, and so on (Shahrokhi-Shahraki et al., 2021). Through the coo- perative activities of electrostatic attraction, ion exchange, and physical adsorption, lead and copper ions were adsorbed onto EDTA-functionalized bamboo activated carbon (Lv et al., 2018). Adsorption of cadmium ions to KOH/ZnCl2 modified activated carbon was mainly due to physical adsorption and pore diffusion (Nayak et al., 2017). Lead and cadmium ions can be adsorbed onto magnetic activated carbon with improved electrostatic attraction and surface complexation (Zhang et al., 2021).
The oxidation of activated carbon is an easy way to enhance reactivity (Guedidi et al., 2017). A nitrogen or oxygen functional group is created on the surface of activated carbon by adding oxygen atoms, allowing for the adsorption of organic contaminants as well as heavy metals. (Kim et al., 2012). Oxidized activated carbon can also be used as a base plate to introduce new functional groups. Functional groups containing oxygen atoms that may exist on the activated carbon surface can include carbonyl, phenolic, carboxyl, lactone groups, and so on (Liu et al., 2007). Hydrogen ions in those functional groups can also be substituted with sodium ions, which can affect the adsorption of heavy metal ions (Ania and Bandosz, 2006; Karnib et al., 2014). Few researchers investigated the adsorption of various heavy metals with and without sodium ions onto surface-oxidized activated carbon. A better understanding will lead to better applications of modified activated carbon materials. Therefore, the objective of this research was to investigate the effects of sodium ions on the adsorption of lead, zinc, and cadmium onto surface oxidized activated carbon.
2.1. Materials
The coal-based granular activated carbon in this study were purchased from Calgon (USA). Nitric acid (HNO3) and hydrochloric acid (HCl) were purchased from Sigma Aldrich (USA). Sodium hydroxide was purchased from SHOWA (Japan). Analytical grade reagents were all utilized directly without further purification. Deionized (DI) water was used to make chemical solutions (18 MΩ cm). A blender was used to render the granular activated carbon into powder. The powders were then passed through a standard 100 to 200 mesh sieve to yield a particle size of 0.15 to 2 mm. The powders were subsequently washed with DI water to remove impurities and dried 60oC for 24 hours to produce washed activated carbon (C-W). C-W (10 g) and 1 L of 5 M HNO3 were added to a brown volumetric flask (1 L) and stirred at room temperature for 24 hours. The resultant activated carbon was washed several times with DI water until the pH became neutral, was centrifuged at 6000 rpm for 15 minutes, and was dried at 80oC for 24 hours to yield the oxidized activated carbon (C-O-5M). The C-O-5M was stirred in 1 L of 1 M sodium hydroxide (NaOH) at 60oC for 3 hours. Finally, the resultant activated carbon was washed again, centrifuged, and dried to yield the sodium-substituted oxidized activated carbon (C-Na).
2.2. Characterization
The physical shape of the activated carbon was confirmed with a scanning electron microscope (SEM) and energy dispersive spectroscopy (EDS) using a FEI Verios G4UC. Trace amounts of synthetic microparticles were sprayed onto carbon tape and transferred to an SEM grid and dried in an oven at 60oC for one day. Then, in order to prevent movement of the microparticles, a platinum coating was applied for 1 minute before the analysis. Fourier transformed infrared (FT-IR) spectroscopy with a PerkinElmer UATR Spectrum Two was used to confirm the chemical structure of the modified stabilizer. The exact elemental content of the functional groups was confirmed through X-ray photoelectron spectroscopy (XPS) using K-Alpha (Thermo Fisher Science). The zeta potential was used to measure the electrostatic interaction between ions and adsorbents by measuring surface charge. The zeta potential before and after activated carbon modification and heavy metal adsorption was measured by analyzing the supernatant with an electro-highlight scattering spectrophotometer (Perkin Elmer). The BET analyses were done at 77 K using N2 adsorption-desorption. All samples were degassed for three hours at 100oC with an argon gas flow using around 0.1 g of each sample. The Barrett-Joyner-Halenda (BJH) method was applied to compute the pore size distribution from the desorption branches of the nitrogen isotherms.
2.3. Adsorption experiments
Adsorption experiments were carried out at 25oC in polypropylene conical tubes. To evaluate the efficiency of adsorption, the concentration of heavy metals(lead, zinc, and cadmium) was prepared from 1 to 10 ppm, respectively. For investing adsorption efficiency in modified activated carbons, each heavy metal was added together into 50 ml of DI water to create a heavy metal solution. The pH of the solution was adjusted to 7 with HCl and NaOH for optimal conditions. The target solutions were agitated with different activated carbon powder doses ranging from 1 to 500 mg∙ L-1 in a rotary shaker at 50 rpm for 24 hours. The samples were immediately removed from the rotary shaker and separated via centrifugation.
3.1. Physicochemical characteristics of activated car-bons
The morphology of activated carbon can be seen in Fig. 1. With oxidation, the surface of the activated carbon (C-O-5 M and C-Na) became more porous. There was no significant difference in shape between C-O-0.5M and C-Na. Fig. 2 shows an intensive distribution of 2 nm meso- pores in all three activated carbons. This pore size was also confirmed from the BET analysis in Table 1. The specific surface area of the Calgon activated carbon before modi- fication was 1041 m2∙g-1. The specific surface area of the oxidized activated carbon decreased to 829.67 m2∙g-1 due to the partial blocking of mesopores (El-Hendawy, 2003). The overall specific surface area of activated carbon with sodium decreased to a greater extent than that of activated carbon with only oxygen functional groups (792.98 m2∙g-1). This was due to the larger atomic size of sodium. The specific surface area and cumulative pore volume of the activated carbon were decreased from C-W to C-O-5M. This was due to partial destruction of the micropores by the effective attack of HNO3 (El-Hendawy, 2003). Those of C-Na were reduced to a greater extent than that of C-O-5M. This was due to the larger atomic size of sodium.
The EDS results in Table 2 indicated that more oxygen functional groups were attached to the surface after oxidization using HNO3. Treatment with NaOH produced sodium atoms on the surface of the oxidized activated carbon. The atomic ratio and functional groups were con- firmed in the XPS results of Fig. 3. The functional groups of C-C/C-H (284.78 eV), C-O(H) (285.98 eV), C=O (287.08 eV), and O-C=O (288.68 eV) were identified. These corre- sponded to phenolic (C-O(H)), carbonyl (C=O), and carboxyl (O-C=O) groups, respectively (Guo et al., 2018). Additionally, Na1s and NaKL1 peaks were identified at 1071.88 eV and 502.08 eV, respectively, confirming a new Na-O bond.
3.2. Adsorption characteristics of heavy metals by modified activated carbons
The adsorption of heavy metals was performed and fitted into two different isotherm models. Langmuir and Freun- dlich models were fitted to predict the equilibrium para- meters. The Langmuir model with homogeneous adsorption of target materials is (Liu, 2006):

where Qe is the concentration of adsorbed heavy metals at an equilibrium concentration (mg∙g-1), Ce is the equilibrium concentration (mg∙L-1), Qmax is the maximum adsorption capacity (mg∙g-1), and KL Is the Langmuir constant, which is related to the energy of adsorption (L∙mg-1). The Freundlich isotherm model is (Sheindorf et al., 1981):

where Qe is the concentration of adsorbed heavy metals ions at equilibrium (mg∙g-1), KF is the Freundlich constant (mg∙L-1), and n is the heterogeneity factor. Both are the constants related to the adsorption capacity and intensity of solute on the adsorbent.
This indicated all activated carbons showed characteris- tics of single-layer heavy metal adsorption. In the Freun- dlich model, the 1/n value represents the adsorption strength between particles and contaminants. The adsorbent is generally known to be easy to adsorb when the value is between the range of 0.1 to 0.5, and it is known to be poorly absorbed if it is 2 or more. Therefore, Freundlich model was suitable for simulation of adsorption trend. However, the correlation coefficient (R2) values of Lang- muir model were much higher than those of Freundlich (Table 3), and then it is better fit to the Langmuir model.
For C-Na, the maximum adsorption capacity estimated with the Langmuir isotherm model was the highest at 4.84, 3.82, and 18.99 mg∙g-1 for Zn, Cd, and Pb, respectively. The KF of the Freundlich model was also the highest for Zn, Cd, and Pb at 2.18, 2.3, and 10.26 mg∙g-1, respectively, indi- cating that C-Na had the higher adsorption capacity for heavy metals than C-O-5M and C-W (Table 3). Based on the zeta-potential measurements before and after heavy metal adsorption, the adsorption of heavy metals on surface modified activated carbons could be explained (Table 4).
The higher the adsorbed concentration onto the surface-modified activated carbon was, the lower was the zeta potential value. Among the activated carbons, C-Na had the lowest zeta potential and C-W had the highest. The surface-modified activated carbon had a lower zeta potential compared to that of activated carbon due to the carboxyl groups and sodium in the surface-modified activated carbon. The XPS results (Fig. 4) also suggested that the surface-modified activated carbon had more oxygen functional groups compared to that of pure activated carbon. Due to electrostatic interactions between positively charged heavy metals and the negatively charged surface of the activated carbon, the heavy metal adsorption increased in surface-modified activated carbon. Fig. 4 showed the ratio of func- tional groups and oxygen present in the modified activated carbon before and after the adsorption of heavy metals. Before modification, the ratio of oxygen atoms in activated carbon was 8.05%. After oxidizing the surface and modifi- cation with sodium, the proportion of oxygen atoms increased to 15.48% and 13.23%, respectively. Accordingly, the overall proportion of oxygen functional groups also increased. After the adsorption of heavy metals, the ratio of oxygen atoms decreased to 7.45, 15, and 12.8% in C-W, C-O, and C-Na, respectively. The carboxyl groups decreased after heavy metal adsorption, but the ratio of carbonyl groups to hydroxyl groups was increased. These results showed that the main mechanism of heavy metal adsorption was that the ion exchange between H+ of the carboxyl group and the heavy metals.
With the introduction of additional sodium atoms, 5.5% of sodium atoms were generated as shown in Fig. 4. Neutralization of the weakly acidic surface functional groups caused the exchange of H+ of carboxylic acid with Na+ as:
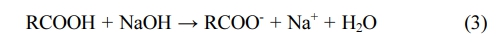
The ratio of carboxyl groups decreased while the ratio of C=O was increased. The weakly acidic O-H phenol reacted with the strong base of NaOH reduced the ratio (Jia and Thomas, 2000). The Na+ of carboxylate was ion-exchanged with heavy metals and it affected heavy metal adsorption. Therefore, the COONa functional group in Na+ substituted activated carbon yielded better ion exchange for heavy metals than COOH in activated carbon after acid treatment. This was because Na+ has less binding energy to COO- than H+. It was easily dissociated and the was the reason why ion exchange became easier (Zhang et al., 2018).
The adsorption of heavy metals was changed with the pH (Fig. 5). The adsorption tended to be lower in acidic and alkali conditions. These results were related to the ion exchange capacity in relation to charge of the surface. The activated carbon surface showed a negative zeta potential under neutral conditions, but it was changed to positive under acidic conditions. The electrostatic adsorption of heavy metals did not easily occur under acid conditions. It showed a lower adsorption under acidic conditions than at a neutral pH (Hu et al., 2005).
Heavy metals exhibit different speciation depending on pH changes at alkali pH conditions. Cd(OH)2 is a dominant species under alkali conditions greater than pH 8.8 (Lee et al., 2012). Similarly, Cd, Pb(OH)2, and Zn(OH)2 are domi- nant species at pHs higher than pH 9.4 (Cruz-Lopes et al., 2021). The decrease in adsorption of heavy metals under alkali conditions resulted from the formation of heavy metal precipitations, which interfered with adsorption and ion exchange on the surface of activated carbon. The most appropriate pH conditions for heavy metal adsorption was between pH 5 and 8 in surface modified activated carbon.

|
Fig. 1 EM images of (a) C-W, (b) C-O-0.5M, and (c) C-Na. |

|
Fig. 2 Characteristics of pores determined with the BET analysis. |

|
Fig. 3 XPS spectra of surface modified activated carbon. |

|
Fig. 4 Changes in functional groups, oxygen, and sodium content before and after adsorption of heavy metals. |

|
Fig. 5 Changes in adsorption efficiency of Zn, Cd and Pb from pH 3 to 10 by C-Na. |
|
Table 3 Langmuir and Freundlich isotherm parameters for Zn, Cd and Pb sorption by C-W and C-Na |

The adsorption efficiency in this research was high in the order of C-Na (1 M NaOH after 5 M HNO3 treatment) > C-O-5M (treatment of 5M HNO3) > C-W (non-treatment). Sodium atoms with oxygen functional groups were closely related to ion exchange with heavy metals. This was also shown in the BET, XPS, and zeta potential analysis results. The oxygen atoms and oxygen functional groups in activated carbon increased after oxidation. Reduced carboxyl group content affected heavy metal adsorption. The adsorption to C-Na, which contained the most oxygen functional groups and Na+ was higher than C-O-5M. This suggested that ion exchange with Na+ in carboxyl groups effectively improved heavy metal adsorption rather than electrostatic adsorption and hydrogen ion exchange. The most appropriate pH condition for heavy metal adsorption was neutral for heavy metal adsorption to surface modified activated carbon.
This work was supported by the Korea Environmental Industry & Technology Institute (KEITI) through Aquatic Ecosystem Conservation Research Program, funded by the Korea Ministry of Environment (MOE) (No. 202100304 0004).
There are no conflicts to declare.
- 1. Ania, C.O. and Bandosz, T.J., 2006, Sodium on the surface of activated carbons as a factor enhancing reactive adsorption of dibenzothiophene, Energy Fuels, 20(3), 1076-1080. https://doi.org/10.1021/ef050425c
-

- 2. Babel, S. and Kurniawan, T.A., 2003, Low-cost adsorbents for heavy metals uptake from contaminated water: a review, J. Hazard. Mater., 97(1-3), 219-243. https://doi.org/10.1016/S0304-3894(02)00263-7
-

- 3. Ciobanu, G., Harja, M., Rusu, L., Mocanu, A.M., and Luca, C., 2014, Acid black 172 dye adsorption from aqueous solution by hydroxyapatite as low-cost adsorbent, Korean J. Chem. Eng., 31, 1021-1027. https://doi.org/10.1007/s11814-014-0040-4
-

- 4. Cruz-Lopes, L.P., Macena, M., Esteves, B., and Guiné, R.P.F., 2021, Ideal pH for the adsorption of metal ions Cr6+, Ni2+, Pb2+ in aqueous solution with different adsorbent materials, Open Agric., 6(1), 115-123. https://doi.org/10.1515/opag-2021-0225
-

- 5. El-Hendawy, A.N.A., 2003, Influence of HNO3 oxidation on the structure and adsorptive properties of corncob-based activated carbon, Carbon, 41(4), 713-722. https://doi.org/10.1016/S0008-6223(03)00029-0
-

- 6. Guedidi, H., Reinert, L., Soneda, Y., Bellakhal, N., and Duclaux, L., 2017, Adsorption of ibuprofen from aqueous solution on chemically surface-modified activated carbon cloths, Arab. J. Chem.,10(2), S3584-S3594. https://doi.org/10.1016/j.arabjc.2014. 03.007
-

- 7. Guo, S., Dan, Z., Duan, N., Chen, G., Gao, W., and Zhao, W., 2018, Zn (II), Pb (II), and Cd (II) adsorption from aqueous solution by magnetic silica gel: preparation, characterization, and adsorption, Environ. Sci. Pollut. Res., 25, 30938-30948. https://doi.org/10.1007/s11356-018-3050-7
-

- 8. Hu, H., Yu, A., Kim, E., Zhao, B., Itkis, M.E., Bekyarova, E., and Haddon, R.C., 2005 Influence of the zeta Potential on the dispersability and purification of single-walled carbon nanotubes, J. Phys. Chem. B, 109(23), 11520-11524. https://doi.org/10.1021/jp050781w
-

- 9. Ismadji, S., Sudaryanto, Y., Hartono, S.B., Setiawan, L.E.K., and Ayucitra, A., 2005, Activated carbon from char obtained from vacuum pyrolysis of teak sawdust: pore structure development and characterization, Bioresour. Technol.,96(12), 1364-1369.https://doi.org/10.1016/j.biortech.2004.11.007
-

- 10. Jia, Y.F. and Thomas, K.M., 2000, Adsorption of cadmium ions on oxygen surface sites in activated carbon, Langmuir, 16(3), 1114-1122.https://doi.org/10.1021/la990436w
-

- 11. Karnib, M., Kabbani, A., Holail, H., and Olama, Z., 2014, Heavy metals removal using activated carbon, silica and silica activated carbon composite, Energy Procedia, 50, 113-120. https://doi.org/10.1016/j.egypro.2014.06.014
-

- 12. Kim, D., Kim, C., Chun, B., and Park, J.W., 2012, Enhanced heavy mmetal sorption by ssurface-oxidized activated carbon does not affect the PAH sequestration in sediments, Water Air Soil Pollut., 223, 3195-3206. https://doi.org/10.1007/s11270-012-1101-0
-

- 13. Kim, D., Jung, Y., Kwon, S., and Park, J.W., 2011, Adsorption of cadmium (II) from aqueous solutions by thiol-functionalized activated carbon, Water Supply, 11(1), 61-66. https://doi.org/10.2166/ws.2011.009
-

- 14. Lee, S.M., Laldawngliana, C., and Tiwari, D., 2012, Iron oxide nano-particles-immobilized-sand material in the treatment of Cu(II), Cd(II) and Pb(II) contaminated waste waters, Chem. Eng. J., 195-196, 103-111. https://doi.org/10.1016/j.cej.2012.04. 075
-

- 15. Liu, S.X., Chen, X., Chen, X.Y., Liu, Z.F., and Wang, H.L., 2007, Activated carbon with excellent chromium(VI) adsorption performance prepared by acid-base surface modification, J. Hazard. Mat., 141(1), 315-319. https://doi.org/10.1016/j.jhazmat. 2006.07.006
-

- 16. Liu, Y., 2006, Some consideration on the langmuir isotherm equation, Colloids Surf. A Physicochem. Eng. Asp., 274(1-3), 34-36.https://doi.org/10.1016/j.colsurfa.2005.08.029
-

- 17. Lv, D., Liu, Y., Zhou, J., Yang, K., Lou, Z., Baig, S.A., and Xu, X., 2018, Application of EDTA-functionalized bamboo activated carbon (BAC) for Pb(II) and Cu(II) removal from aqueous solutions, Appl. Surf. Sci., 428, 648-658. https://doi.org/10.1016/j.apsusc.2017.09.151
-

- 18. Nayak, A., Bhushan, B., Gupta, V., and Sharma, P., 2017, Chemically activated carbon from lignocellulosic wastes for heavy metal wastewater remediation: Effect of activation conditions, J. Colloid. Interface. Sci.,493, 228-240. https://doi.org/10.1016/j.jcis.2017.01.031
-

- 19. Shahrokhi-Shahraki, R., Benally, C., El-Din, M.G., and Park, J., 2021, High efficiency removal of heavy metals using tire-derived activated carbon vs commercial activated carbon: Insights into the adsorption mechanisms, Chemosphere, 264(1), 128455. https://doi.org/10.1016/j.chemosphere.2020.128455
-

- 20. Sheindorf, C., Rebhun, M., and Sheintuch, M., 1981, A freundlich-type multicomponent isotherm, J. Colloid. Interface. Sci., 79(1), 136-142. https://doi.org/10.1016/0021-9797(81)90056-4
-

- 21. Wang, X., Cheng, H., Ye, G., Fan, J., Yao, F., Wang, Y., Jiao, Y., Zhu, W., Huang, H., and Ye, D., 2022, Key factors and primary modification methods of activated carbon and their application in adsorption of carbon-based gases: A review, Chemosphere, 287(2), 131995. https://doi.org/10.1016/j.chemosphere.2021.131995
-

- 22. Zhang, X.F., Feng, Y., Wang, Z., Jia, M., and Yao, J., 2018, Fabrication of cellulose nanofibrils/UiO-66-NH2 composite membrane for CO2/N2 separation, J. Membr. Sci., 568, 10-16. https://doi.org/10.1016/j.memsci.2018.09.055
-

- 23. Zhang, Z., Wang, T., Zhang, H., Liu, Y., and Xing, B., 2021, Adsorption of Pb(II) and Cd(II) by magnetic activated carbon and its mechanism, Sci. Total. Environ., 757, 143910. https://doi.org/10.1016/j.scitotenv.2020.143910
-

 This Article
This Article
-
2023; 28(6): 16-23
Published on Dec 31, 2023
- 10.7857/JSGE.2023.28.6.016
- Received on Sep 27, 2023
- Revised on Oct 17, 2023
- Accepted on Nov 17, 2023
 Services
Services
- Abstract
1. introduction
2. materials and methods
3. results and discussion
4. conclusions
- Acknowledgements
- Conflicts of Interest
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Jae-Woo Park
-
Department of Civil and Environmental Engineering, Hanyang University, Seoul 04763, Korea
- E-mail: jaewoopark@hanyang.ac.kr








