- Geochemical Characteristics and Assesment of Nitrate Nitrogen in Groundwater in Yanggu-Gun, Gangwon-Do in Korea
Won Gyu Choi*
Department of New Energy and Mining Engineering, SangJi University
An analysis of groundwater quality is significant for monitoring and
managing water contamination and groundwater system. For the purpose of those,
the geochemical characteristics of groundwater were studied over the concern
for water quality, water type and origin of nitrate nitrogen. Total colony
counts were detected in 11 out of 20
samples, and the average value was 31.73 CFU/ml. Range and average of NO3-N
concentrations were 0.9~24.0 mg/L and 8.3 mg/L. All groundwater types were found to be Ca2+-HCO3-. The range and average of NO3-N were
0.2~17.4 mg/L and 8.7 mg/L, and those
of δ15N were 1.7~8.9‰, and 5.0‰. Careful consideration is required
for evaluating the origin of nitrogen when NO3-N concentration is
low. In general, noticeable difference between rockbed and alluvial water was
not found. The ranges of nitrate origins by chemical fertilizer, livestock
manure and domestic sewage, and natural soil were 29.6~76.4%, 14.2~58.9% and
2.6~7.0%, and the average values of those were 57.4%, 37.4%, and 5.3%, respectively. Origin of nitrate was
affected by more chemical fertilizer than the other parameters. Rockbed water was more affected by chemical fertilizer than alluvial
water.
Keywords: Groundwater quality, Water type, Nitrate nitrogen, Nitrogen Isotope, Origin of nitrogen
An analysis
of groundwater quality is indispensable for monitoring and managing water
contamination and groundwater system. For the purpose of those, the geochemical
characteristics of groundwater in Haean-Myun, Yanggu-Gun, Gangwon-Do in Korea
were studied over the concern for water quality, water type and origin of
nitrate nitrogen. Water samples were collected from 22 rockbed and 11 alluvial
wells. The analysis was categorized by general items, anions and cations, and
isotopic fractionation of nitrogen.
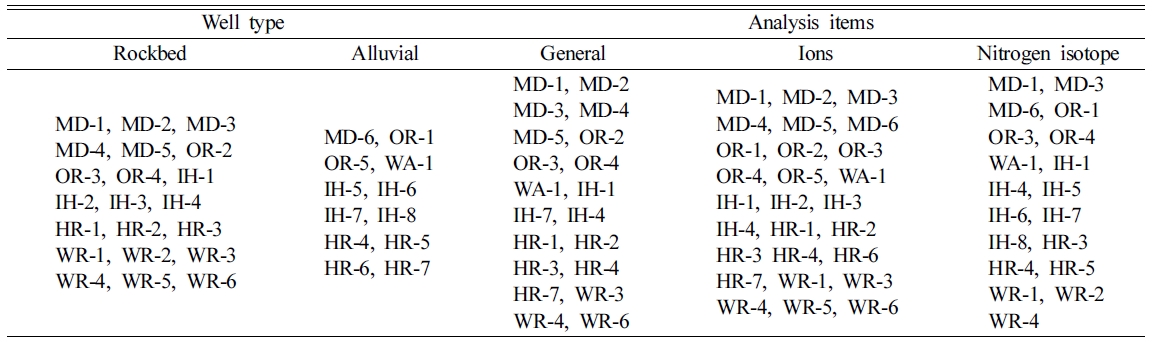
Well type
and analysis items for 33 wells were shown in Table 1. For analyzing isotopic
fractionation of nitrogen (δ15N), samples from 8 alluvial and 11
rockbed wells were selected by reference data provided by Korea Rural Community
Corporation. General analysis items include total colony counts, total
coliforms, pH, EC and NO3-N and Ca2+, K+, Mg2+,
Na+, Cl-, SO42-, CO32-, and HCO3- ions, and isotope of nitrogen were analyzed.
Water
samples for analysis were classified in 3 categories; general items, cation and
anion, and nitrogen isotope. 1~2 L of groundwater was sampled
approximately after 5 minutes pumping from the well to obtain fresh
groundwater. pH and EC were measured in-situ according to Experimental
Standards of Water Pollutants (Ministry of Environ- ment, 2001). And water samples, exclusive
of total coliforms analysis, were pretreated and refrigerated to be delivered
to laboratory. Multiple tube fermentation technique is applied to analyze total
coliforms (Korean standard method of drinking water quality test (ES 05703.1a).
For the cation analysis, in-situ filtering was carried out using 0.45 mm membrane filters, and HNO3 was added to
maintain pH below 2. For anion and nitrogen isotope analysis, the same kind of
filters were used. Ion concentration was analyzed ICP-OES (Vista-MPX, Varian)
and IC (761, Metrohm) according to SW-846 6010A (EPA) and Standard Methods 4110
(AWWA, 18th Ed., 1992), respectively. For analysis of isotopic fractionation of
nitrogen (δ15N), 500 ml of pretreated, airtight and
refrigerated samples were delivered to national instrumentation center for
environmental management (NICEM) in Seoul national university.
Origin of
nitrogen in groundwater has been investigated by many researchers (Power et
al., 1974; Boyce et al., 1976; Holloway et al., 1998; Kreiter et al., 1978). NO3-N
contamination of goundwater is attributed by chemical fertilizer, livestock
manure and domestic sewage, and natural soil (Oh and Hyun, 1997; Choi et al.,
2003). And natural nitrate is associated with rock type, natural and
atmospheric environments, and other parameters (Power et al., 1974; Mike Lowe
and Janae Wallace, 2001). And nitrogen concentration can also be affected by
biochemical and biological transformation. Because, these processes can
influence the release of nitrogen in bedrock into ground water (Holloway and
Smith, 2000). The relationship between nitrate nitrogen (NO3-N) and
isotopic fractionation of nitrogen (δ15N) was analyzed to estimate
origin of nitrogen.
Two types of
hydrogen stable isotopes, 14N and 15N, exist in nature,
and isotopic fractionation of nitrogen (δ15N) can be obtained by
quantified 15N and 14N using equation below.
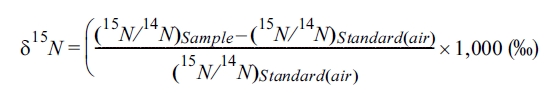
Higher δ15N
implies that heavier 15N exists more than 14N while lower
δ15N implies that 14N exists more than 15N in
the sample. To evaluate the nitrate contamination source in groundwater using δ15N
technique, it is more reasonable to use correlation between the NO3-N
concentrations and δ15N. From this technique, δ15N ranges
of chemical fertilizer, livestock manure and domestic sewage, and natural soil
are –4 ~ +4‰, –10 ~ +22‰, +6 ~ +10‰ and
–4 ~ +8 ‰, respectively (Heaton, 1986, Komor and Anderson, 1993). The
component ratio of nitrate nitrogen from different sources can be estimated by
the following relations (Nakanishi, 1995; Yamamoto et al., 1995; Jeong, 2003).
Sources of nitrate nitrogen using δ15N can be estimated by following
relations.
- W = X + Y
+ Z
- aW = bX +
cY + dZ
where,
W: NO3-N concentration in groundwater
(mg/L)
X: NO3-N concentration originated by chemical
fertilizer (mg/L)
Y: NO3-N concentration originated by
livestock manure and
domestic sewage (mg/L)
Z: NO3-N concentration originated by
natural soil (mg/L)
a: δ15N of NO3-N in
groundwater
(‰)
b: δ15N of NO3-N by
chemical fertilizer
(‰)
c: δ15N of NO3-N by
livestock manure and domestic sewage (‰)
d: δ15N of NO3-N by natural
soil (‰)
Concentration
of NO3-N originated by natural soil was ranging from 0.45 mg/L
to 0.9 mg/L, and the minimum of 0.45 mg/L was applied as reported by
Korea Rural Community Corporation (KRC). And δ15N values of origin
from chemical fertilizer, livestock manure and domestic sewage, and natural
soil were 0‰, 14‰ and 1.8‰ provided by KRC.
3.1. General items analysis
The
experimental results of general items at 20 water wells were shown in Table 2.
The range and average value of pH were 6.00~7.12 and 6.76, and those of
electric conductivity (EC) were 46~398 μS/cm and 237 μS/cm. pH and EC
measurements between rockbed and alluvial water showed no significant
difference. Total colony counts was not detected 9 out of 20 samples, and the
average value of total colony counts detected was 21.2 CFU/ml that is
within Korean groundwater quality standard of less than 100 CFU/ml. And
the total coliforms were counted in MD-5, OR-3, WA-1 and HR-7 out of 20
samples. NO3-N concentrations were in the ranges of 0.9~
24.0 mg/L, and the average value was 8.3 mg/L. It is noted that
nitrate nitrogen concentration of IH-1 was 24.0 mg/L that exceeded above
water quality standard. And average NO3-N values of rockbed and
alluvial water were 7.9 mg/L and 10.6 mg/L. In general, noticeable
results were not identified.
3.2. Ion analysis
Cation and
anion concentrations from 23 rockbed and 7 alluvial water were analyzed, and
summarized in Table 3.
From the
results, noticeable concentrations difference between rockbed and alluvial
water were not identified. And from groundwater type analysis using the piper
diagram, all water type was identified to be Ca2+-HCO3- that implies typical type of fresh water (Fig. 1).
3.3. Assesment of nitrate nitrogen
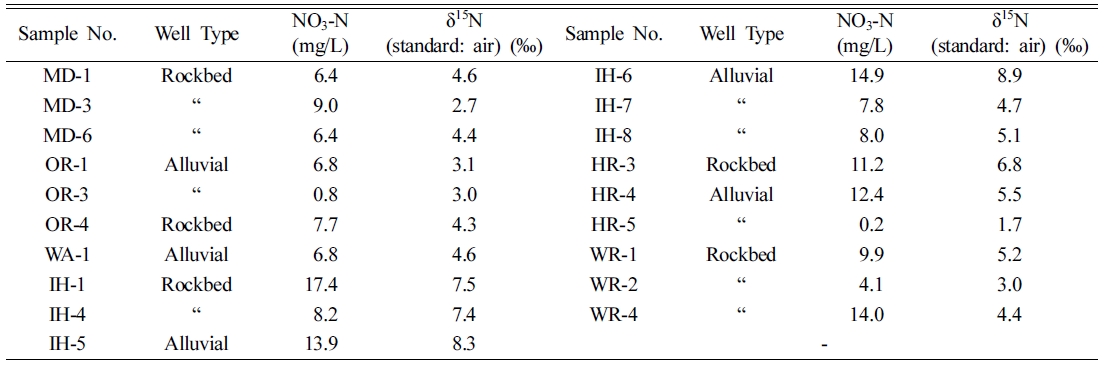
The results
of NO3-N and δ15N analysis from 11 rockbed and 8 alluvial
water samples were summarized in Table 4. The ranges and average values of NO3-N
and δ15N were 0.2~17.4 mg/L and 8.7 mg/L and 1.7~8.9‰, and
5.0‰, respectively. The averages of NO3-N and δ15N in
rockbed and alluvial water were 8.7 mg/L and 4.7‰ and 8.8 mg/L and
5.4‰, and noticeable difference between them was not found.
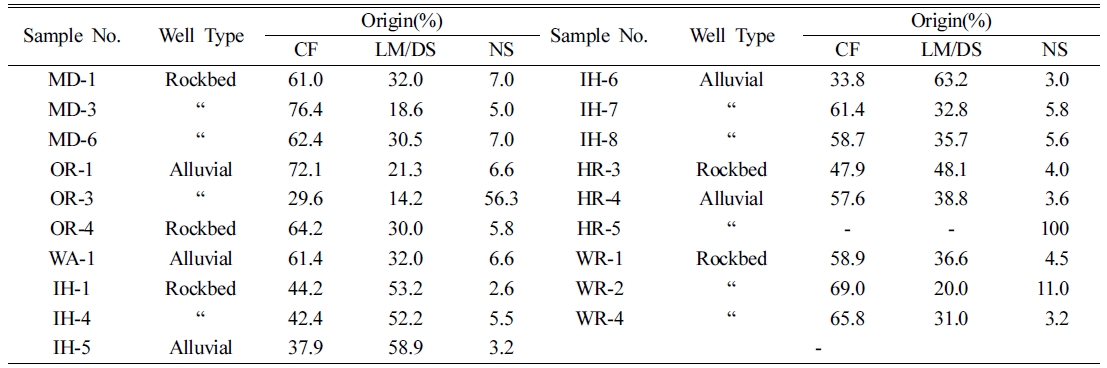
The origins
of nitrate nitrogen by chemical fertilizer, livestock manure and domestic
sewage, and natural soil were summarized in Table 5.
The results
of 56.3% at OR-3 and 100% at HR-5 were evaluated. Distinctly lower NO3-N
concentrations of 0.8 mg/L and 0.2 mg/L from two samples were
noticed. It is presumed that NO3-N can be originated by natural
soil. Careful consideration, hence, is necessary for evaluating the origin of
nitrate nitrogen. The ranges of nitrate origins by chemical fertilizer,
livestock manure and domestic sewage, and natural soil are 29.6~76.4%,
14.2~58.9% and 2.6~ 7.0%, and the average values of those are 57.4%, 37.4% and
5.3%, respectively except from OR-3 and HR-5. Regarding origin of nitrate
nitrogen, chemical fertilizer affects more than livestock manure and domestic
sewage, and natural soil. And the average values of nitrogen origin by chemical
fertilizer, livestock manure and domestic sewage, and natural soil in rockbed
samples were 60.2%, 34.3% and 5.5%, and those of alluvial samples were 53.2%,
41.7% and 5.0%, respectively. It is noted that chemical fertilizer affects
origin of nitrate nitrogen more in rockbed water than alluvial water, while
less affects by livestock manure and domestic sewage.
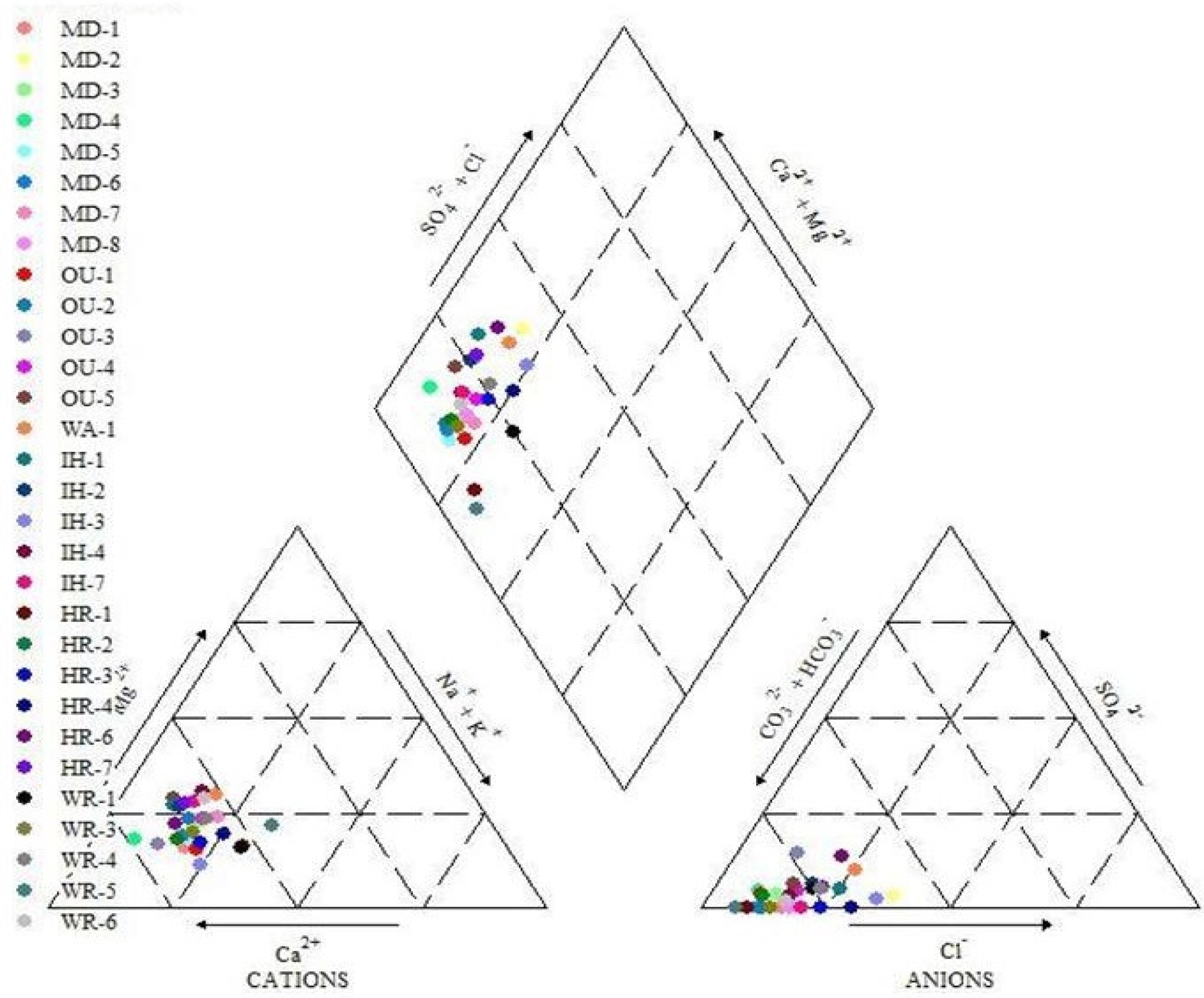
|
Fig. 1 Water type classification using piper diagram. |
|
Table 2 The results of general item analysis |
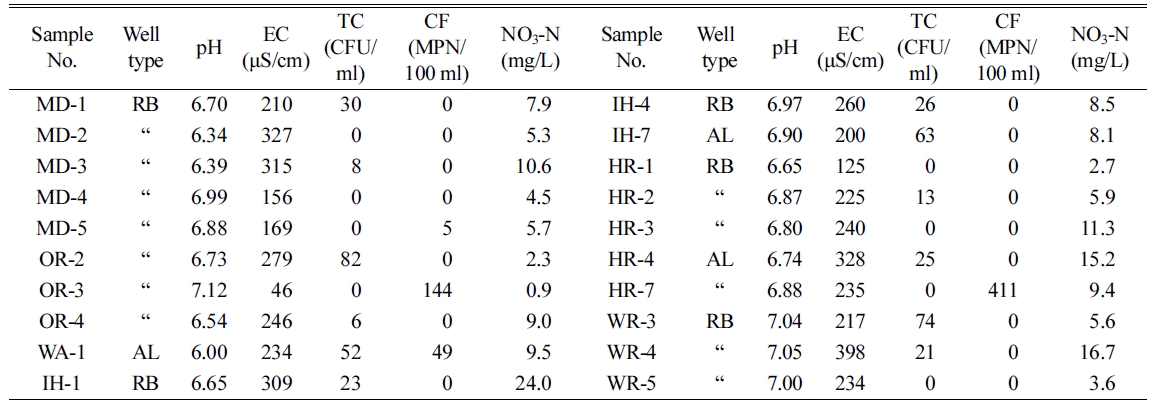
* TC: total colony counts, CF: total coliforms ** Well type RB: Rockbed, AL: Alluvial |
|
Table 3 Ion analysis results of rockbed and alluvial water |
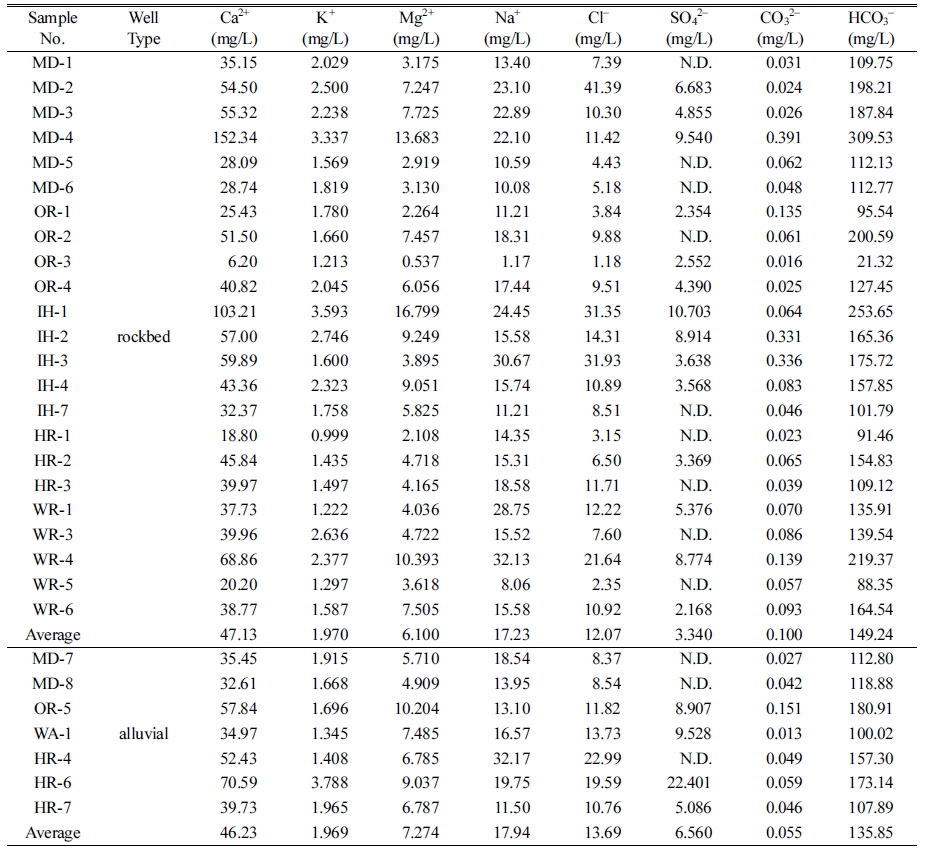
* N.D.: Non-detected |
pH range and
average value were 6.00~7.12 and 6.76, and those of electric conductivity (EC)
were 46~398 μS/cm and 237 μS/cm. pH and EC measurements between
rockbed and alluvial water were not significant. From the results of general
item analysis, total colony counts were detected 11 out of 20 samples, and
average value of was 31.73 CFU/ml. And the total coliforms were counted in
3 out of 20 samples. The ranges of NO3-N concentrations was 0.9~24.0
mg/L, and the average value was 8.3 mg/L. And average NO3-N
values of rockbed and alluvial water were 7.9 mg/L and 10.6 mg/L,
respectively. In general, noticeable results were not found between rockbed and
alluvial water. From the ion analysis, noticeable concentrations difference
between rockbed and alluvial water was not identified. And from groundwater
type analysis using the piper diagram, all water types were identified to be Ca2+-HCO3- that implies typical type of fresh water. The ranges
and average values of NO3-N were 0.2~17.4 mg/L and
8.7 mg/L, and those values of δ15N were 1.7~8.9‰, and 5.0‰. The
averages of NO3-N and δ15N in rockbed and alluvial water
were 8.7 mg/L and 4.7‰ and 8.8 mg/L and 5.4‰, respectively, and
noticeable difference between them was not found. The results of 56.3% of δ15N
at OR-3 (NO3-N 0.8 mg/L) and 100% at HR-5 (NO3-N
0.2 mg/L) were evaluated. It is supposed that distinctly lower NO3-N
concentrations can be caused by natural soil rather than the other factors.
Careful consideration, hence, is required for evaluating the origin of nitrate
nitrogen. The ranges of nitrate origins by chemical fertilizer, livestock
manure and domestic sewage, and natural soil were 29.6~76.4%, 14.2~58.9% and
2.6~7.0%, and the average values of those were 57.4%, 37.4% and 5.3%,
respectively. Chemical fertilizer affects origin of nitrate nitrogen more than
the other origins. Rockbed water are more affected by chemical fertilizer than
alluvial water, while less affected by livestock manure and domestic sewage.
The study can be referenced for basic data and future researches for monitoring
and management of groundwater quality in the region.
This
research was supported by Gangwon Regional Headquarter of Korea Rural Community
Corporation.
- 1. American Public Health Association, Washington, U.S., 1992, Standard Methods for the Examination of Water and Waste Water, AWWA, 18th Ed.
- 2. Boyce, J.S., Muir, John, Edwards, A.P., Seim, E.C., and Olson, R.A., 1976, Geologic nitrogen in Pleistocene loess of Nebraska: J. of Environmental Quality, 5, 93-96.
-

- 3. Jeong, C.H., 2003 Hydrochemistry and Nitrogen and Sulfur Isotopes of Emergency-use Groundwater in Daejeon City, The J. of Engineering Geology, 13(2), 239-256.
- 4. Choi, W.J., Lee, S.M., and Ro, H.M. 2003, Evaluation of contamination sources of groundwater NO3- using nitrogen isotope data: A review, Geosciences Journal, 7(1), 81-87.
-

- 5. Heaton, T.H.E., 1986, Isotropic studies of nitrogen pollution in the hydrosphere and atmosphere, Chemical Geology, 59, 87-102.
-

- 6. Holloway, J.M. and Dahlgren, R.A., 1999, Geologic nitrogen in terrestrial biogeochemical cycling: Geology, 27, 567-570.
-

- 7. Holloway, J.M. and Smith, R.L., 2000, Biogeochemical transformations influencing release and cycling of nitrogen in shale to stream and ground water: Geological Society of America Abstracts with programs, 32(7), A-191.
- 8. Holloway, J.M., Dahlgren, R.A., Hansen, B., and Casey, W.H., 1998, Contribution of bedrock nitrogen to high nitrate concentrations in stream water: Nature, 395, 785-788.
-

- 9. Komor, S.C. and Anderson, H.W., 1993, Nitrogen Isotope as indicators of nitrates sources in Minnesota Sand-Plain aquifer, Ground Water, 31(2), 260-270.
-

- 10. Korea Rural Community Corporation, 2010, Rural groundwater management report
- 11. Korean standard method of drinking water quality test (ES 05703.1a), 2012.
- 12. Kreitler, D.W. and Jones, D.C., 1975, Natural soil nitrate: The cause of the nitrate contamination of ground water in Runnels county, Texas, Ground Water, 13(1), 53-61.
-

- 13. Kreitler, C.W., Ragone, S.E., and Katz, B.G., 1978, N15/N14 Ratios of Gound-Water Nitrate, Long Island, New York, Ground Water, 16(6), 404-409.
-

- 14. Mike Lowe and Janae Wallace, 2001, Evaluation of Potential Geologic Sources of Nitrate Contamination in Ground Water, Ceder Valley, Iron County, Utah With Emphasis on the Enoch Area, Utah Department of Natural Resources, 26-30.
- 15. Nakanishi, Y., 1995, Estimation and verification of origins of groundwater nitrate by using delta 15N values, The Agriculture, Forestry and Fisheries Research Information Technology Center, 544-551.
- 16. Oh, Y.K. and Hyun, I.H., 1997, Estimation of Nitrate-nitrogen Contamination Sources in Cheju Island Groundwater using ¥ä15N Values, J. of the Korean Society of Groundwater Environment, 4(1), 1-4.
- 17. Power, J.F., Bond, J.J., Sandoval, F.M., and Willis, W.O., 1974, Nitrification in Paleocene shale: Science, 183, 1077-1079.
-

- 18. Yamamoto, Y., Park, K.L., Nakanishi, Y., and Kato, S., 1995, Nitrate concentrations and delta super (15)N values of groundwater in the Miyakojima Island, Japanese Journal of Soil and Plant Nutrition, 18-25.
 This Article
This Article
-
2019; 24(6): 26-32
Published on Dec 31, 2019
- 10.7857/JSGE.2019.24.6.026
- Received on Dec 9, 2019
- Revised on Dec 9, 2019
- Accepted on Dec 20, 2019
 Services
Services
- Abstract
1. introduction
2. experiments and assesment
3. results and discussion
4. conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Won Gyu Choi
-
Department of New Energy and Mining Engineering, SangJi University
- E-mail: wgchoi@sangji.ac.kr